Brown Adipose Tissue MRI
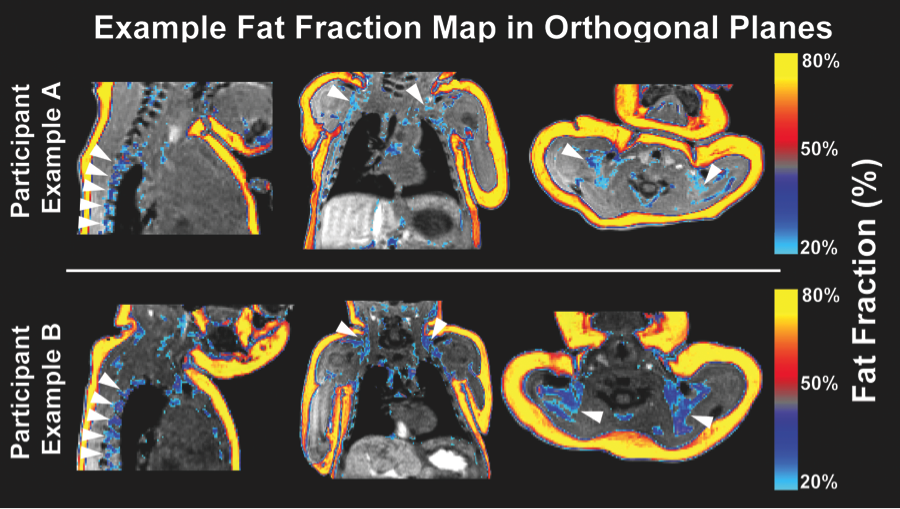
There is a major resurgence of interest in brown adipose tissue (BAT) biology, particularly regarding its determinants and consequences in newborns and infants. Reliable methods for non-invasive BAT measurement in human infants have yet to be demonstrated. The current study first validates methods for quantitative BAT imaging of rodents post mortem followed by BAT excision and re-imaging of excised tissues. Identical methods are then employed in a cohort of in vivo infants to establish the reliability of these measures and provide normative statistics for BAT depot volume and fat fraction. Using multi-echo water-fat MRI, fat- and water-based images of rodents and neonates were acquired and ratios of fat to the combined signal from fat and water (fat signal fraction) were calculated. Neonatal scans (n = 22) were acquired during natural sleep to quantify BAT and WAT deposits for depot volume and fat fraction. Acquisition repeatability was assessed based on multiple scans from the same neonate. Intra- and inter-rater measures of reliability in regional BAT depot volume and fat fraction quantification were determined based on multiple segmentations by two raters. Rodent BAT was characterized as having significantly higher water content than WAT in both in situ as well as ex vivo imaging assessments. Human neonate deposits indicative of bilateral BAT in spinal, supraclavicular and axillary regions were observed. Pairwise, WAT fat fraction was significantly greater than BAT fat fraction throughout the sample (ΔWAT-BAT = 38 %, p<-10(-4)). Repeated scans demonstrated a high voxelwise correlation for fat fraction (Rall = 0.99). BAT depot volume and fat fraction measurements showed high intra-rater (ICCBAT,VOL = 0.93, ICCBAT,FF = 0.93) and inter-rater reliability (ICCBAT,VOL = 0.86, ICCBAT,FF = 0.93). This study demonstrates the reliability of using multi-echo water-fat MRI in human neonates for quantification throughout the torso of BAT depot volume and fat fraction measurements.
Fetal Programming of Energy Homeostasis
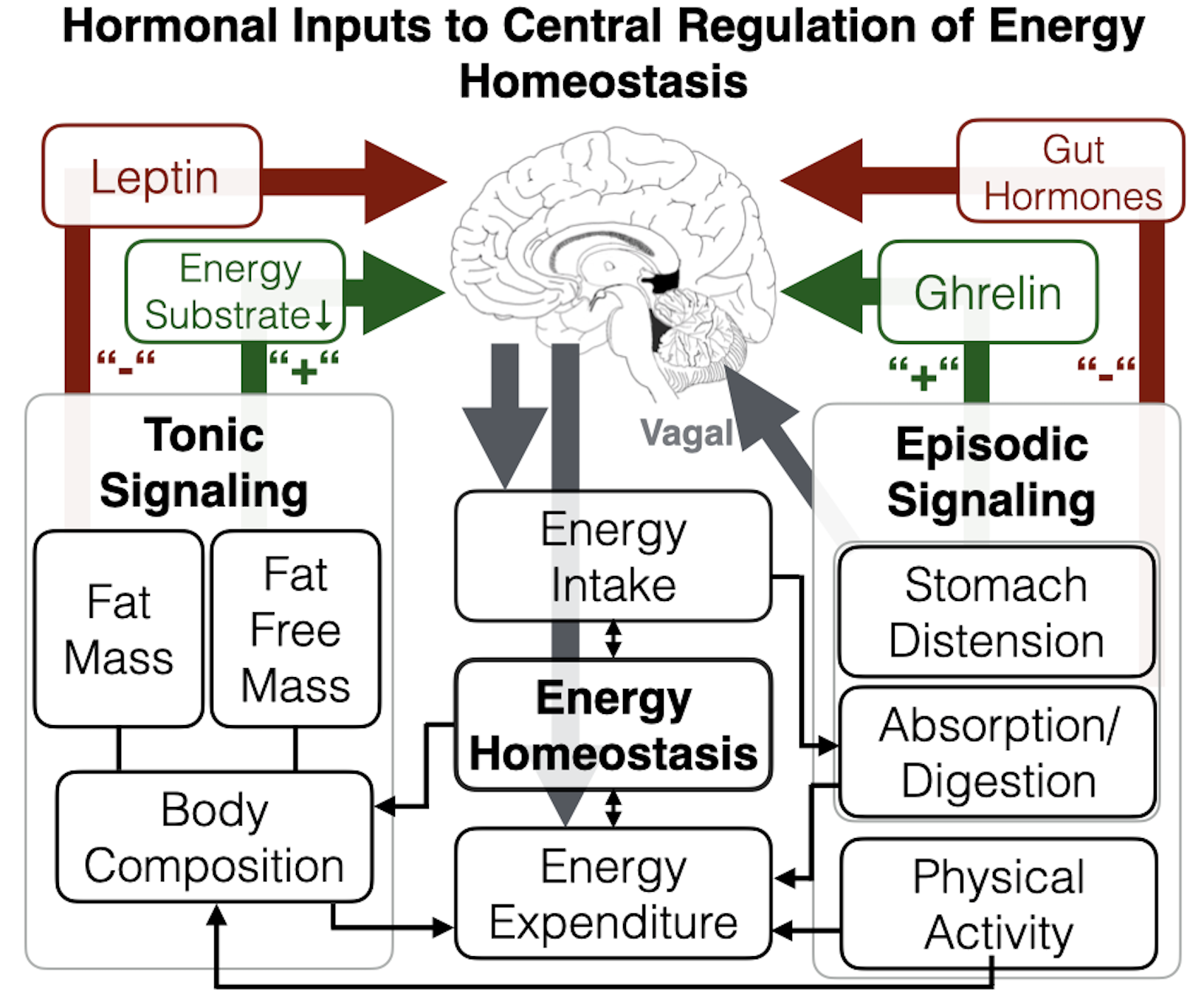
In this paper, we present a transdisciplinary framework and testable hypotheses regarding the process of fetal programming of energy homeostasis brain circuitry. Our model proposes that key aspects of energy homeostasis brain circuitry already are functional by the time of birth (with substantial interindividual variation); that this phenotypic variation at birth is an important determinant of subsequent susceptibility for energy imbalance and childhood obesity risk; and that this brain circuitry exhibits developmental plasticity, in that it is influenced by conditions during intrauterine life, particularly maternal-placental-fetal endocrine, immune/inflammatory, and metabolic processes and their upstream determinants. We review evidence that supports the scientific premise for each element of this formulation, identify future research directions, particularly recent advances that may facilitate a better quantification of the ontogeny of energy homeostasis brain networks, highlight animal and in vitro-based approaches that may better address the determinants of interindividual variation in energy homeostasis brain networks, and discuss the implications of this formulation for the development of strategies targeted towards the primary prevention of childhood obesity.
Newborn Insula Volume and Fat Gain
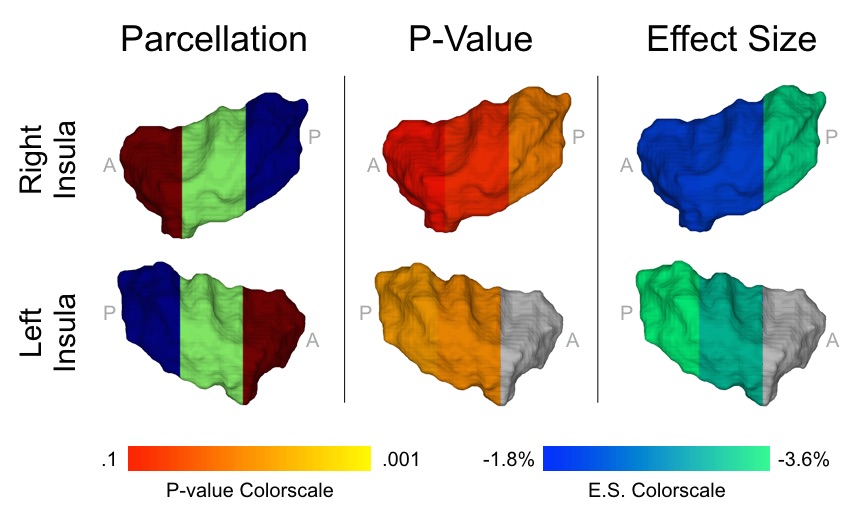
The importance of energy homeostasis brain circuitry in the context of obesity is well established, however, the developmental ontogeny of this circuitry in humans is currently unknown. Here, we investigate the prospective association between newborn gray matter (GM) volume in the insula, a key brain region underlying energy homeostasis, and change in percent body fat accrual over the first six months of postnatal life, an outcome that represents among the most reliable infant predictors of childhood obesity risk. A total of 52 infants (29 male, 23 female, gestational age at birth=39(1.5) weeks) were assessed using structural MRI shortly after birth (postnatal age at MRI scan=25.9(12.2) days), and serial Dual X-Ray Absorptiometry shortly after birth (postnatal age at DXA scan 1=24.6(11.4) days) and at six months of age (postnatal age at DXA scan 2=26.7(3.3) weeks). Insula GM volume was inversely associated with change in percent body fat from birth to six-months postnatal age and accounted for 19% of its variance (β=-3.6%/S.D., P=0.001). This association was driven by the central-posterior portion of the insula, a region of particular importance for gustation and interoception. The direction of this effect is in concordance with observations in adults, and the results remained statistically significant after adjusting for relevant covariates and potential confounding variables. Altogether, these findings suggest an underlying neural basis of childhood obesity that precedes the influence of the postnatal environment. The identification of plausible brain-related biomarkers of childhood obesity risk that predate the influence of the postnatal obesogenic environment may contribute to an improved understanding of propensity for obesity, early identification of at-risk individuals, and intervention targets for primary prevention.